Combustion Modelling
This section on my website is for combustion modelling.
Important notes
1- Please don’t send me an email asking me to send you tutorials, what is available has been uploaded on the website.
2- I would very much appreciate any feedback about the tutorials, and your contribution will be stated in the tutorial, I plan to update the tutorials.
Important notes
1- Please don’t send me an email asking me to send you tutorials, what is available has been uploaded on the website.
2- I would very much appreciate any feedback about the tutorials, and your contribution will be stated in the tutorial, I plan to update the tutorials.
Premixed Combustion Modelling in ANSYS CFX
|
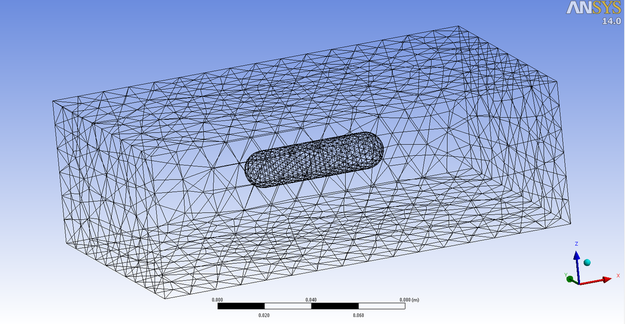
Here is the latest tutorial for premixed Methane combustion modeling. You can download the tutorial by clicking on the right hand side shown image.
Regarding the tutorial geometry you can download the geometry file by click on the mesh image below . It is in para solid format meaning you will just need to read it into Design modeler and meshing can be done automatically. The reason for not using the ANSYS-CFX library was to show the method in dealing with reactive flows and how to set them up. If you can do the tutorial you can work with any reactive flow case relating to chemical engineering. The main intention of having that ball is to form a circulation filed after it and also to form a source of ignition to the mixture. This is done through assigning the ball surface a heat source term. Combustion is encountered in most engineering applications. Ranging from boilers to gas turbines to internal combustion engines…..etc. This tutorial guides the researcher through a step by step process to get a simple combustion done. From this tutorial the user will be able to get the general required guideline to conduct a combustion simulation. |
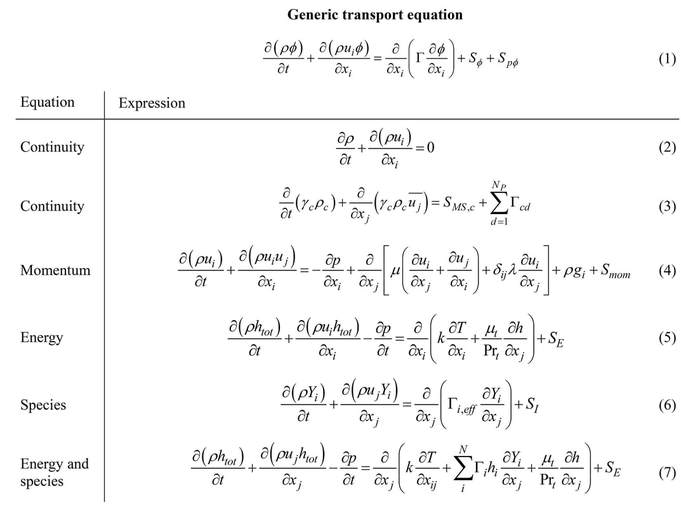
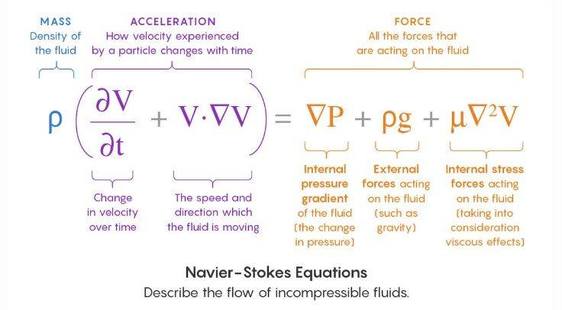
When considering combustion or the case of reactive flow then the 6 equations are required to be solved for the planned calculations, equations from 2 till 7 are extracted from the generic transport equation:
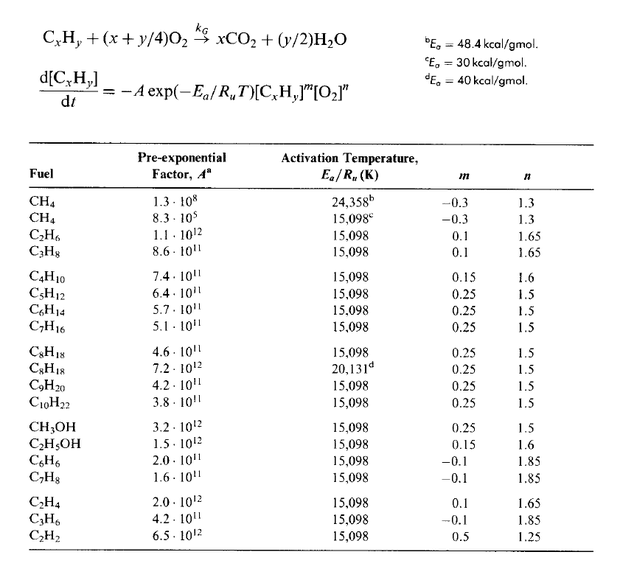
Options for Selecting Other Fuels for the Tutorial
The researcher can use this table to assign other types of fuels not just the Methane fuel used in the tutorial.
Numerical Simulation Validation with Experimental
A video showing a premixed Methane flame available on YouTube. As visible from the video the resulting profile from the video very much mimics the simulation generated data.
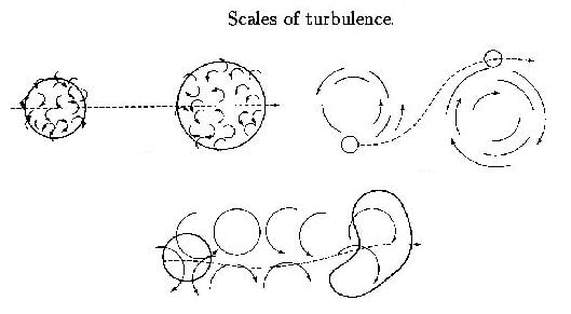
Note: Remember that to convert from ppm to volume fractions you can use 0.01% to 10 000 ppm. Through out any combustion eddies (scales of turbulence) are transported and diffuse within the fluid domain. This is something can also been seen buoyancy and mass transport.
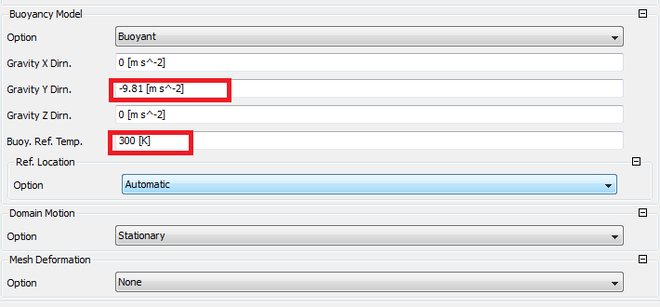
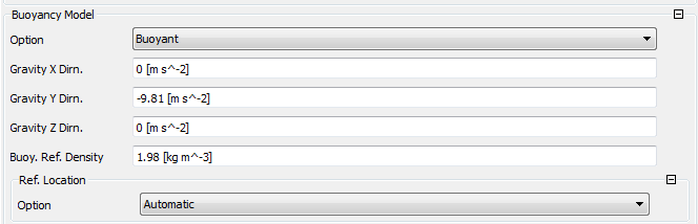
Buoyancy Reference Density or Temperature?
This is some thing that you encounter in diffusion flames, there fore the factor of density change has to be taken into account. Steps on how to find the reference density and to assign the axis of direction.
A good Recommended link that covers the point:
1-http://www.esi-cfd.com/esi-users/buoyancy/
2-http://www.arc.vt.edu/ansys_help/cfx_mod/i1298650.html#CDDCBCHC
3-http://www.arc.vt.edu/ansys_help/cfx_thry/i1299782.html
4-http://en.wikipedia.org/wiki/Boussinesq_approximation_(buoyancy)
5-http://aerojet.engr.ucdavis.edu/fluenthelp/html/ug/node572.htm
6- good read thesis: http://www.legi.grenoble-inp.fr/people/Achim.Wirth/lecnBDF.pdf
1-http://www.esi-cfd.com/esi-users/buoyancy/
2-http://www.arc.vt.edu/ansys_help/cfx_mod/i1298650.html#CDDCBCHC
3-http://www.arc.vt.edu/ansys_help/cfx_thry/i1299782.html
4-http://en.wikipedia.org/wiki/Boussinesq_approximation_(buoyancy)
5-http://aerojet.engr.ucdavis.edu/fluenthelp/html/ug/node572.htm
6- good read thesis: http://www.legi.grenoble-inp.fr/people/Achim.Wirth/lecnBDF.pdf
Assigning the simulation values:
You can also assign the reference quantity as density
Choosing the Nusselt Number for Mass Transfer between Two Gases
The Prandtl number is governed by the temperature field in the cubic domain. Its the ratio of kinematic viscosity and thermal diffusion. Both mentioned parameters are a function of temperature. Remember that: (Pr<< 1 means thermal diffusion dominates),(Pr>> 1 means momentum diffusion dominates) You can calculate your Prandtl number based on the temperature you want. Have a look through the following link for kinematic viscosity values. Considering gases thermal diffusion values can be found in heat transfer books.
Thermally and Calorically Perfect Gas
The importance of this concept is to select the right gas properties. A thermally perfect gas satisfy the following properties:
1- Is in thermodynamic equilibrium.
2- Is not chemically reacting.
3- Has internal energy e, enthalpy h, and specific heat CV that are functions of temperature only and not of pressure.
In a reactive flow case the selected gas properties should be Constant Property Gases at STP.
1- Is in thermodynamic equilibrium.
2- Is not chemically reacting.
3- Has internal energy e, enthalpy h, and specific heat CV that are functions of temperature only and not of pressure.
In a reactive flow case the selected gas properties should be Constant Property Gases at STP.
Coding Combustion Reactions in CFD
|
Youtube link showing the steps for the chemical balancing steps for a combustion process. It is required by the modeler to fully understand these steps and even do them by hand during the simulation setup stage. Hence knowing how to calculate the molecular weight is essential.
|
|
Needed Fundamental Concepts for Combustion
|
What is the meaning of mole and why is it used and what is its relation to the grams units. This is a descriptive video found on YouTube.
|
|
MATLAB Code for Coal Combustion
% Combustion Modelling
% Coal Combustion Example
clc
clear
m_C=84.36;
m_H2=1.89;
m_O2=4.40;
m_N2=0.63;
m_S=0.89;
MW_C=12;
MW_H2=2;
MW_O2=32
MW_N2=28;
MW_S=32;
N_C=m_C/MW_C
N_H2=m_H2/MW_H2
N_02=m_O2/MW_O2
N_N2=m_N2/MW_N2
N_S=m_S/MW_S
x=N_C
y=N_H2
z=N_S
ath=x+0.5*y+z-N_02
w=0.0225+3.76*ath
mprod=x+y+z+w
NCO2=N_C
NH2O=N_H2
NSO2=N_S
NN2=N_N2
MW_CO2=44;
MW_H2O=18;
MW_SO2=64;
MW_N2=28;
YCO2=NCO2/mprod
YH2O=NH2O/mprod
YSO2=NSO2/mprod
YN2=NN2/mprod
Mprod=(N_C*MW_CO2+N_H2*MW_H2O+MW_SO2*NSO2+N_N2*MW_N2)/mprod
mfuel=100;
mair=29*(ath*4.76)
AF=mair/mfuel
% Coal Combustion Example
clc
clear
m_C=84.36;
m_H2=1.89;
m_O2=4.40;
m_N2=0.63;
m_S=0.89;
MW_C=12;
MW_H2=2;
MW_O2=32
MW_N2=28;
MW_S=32;
N_C=m_C/MW_C
N_H2=m_H2/MW_H2
N_02=m_O2/MW_O2
N_N2=m_N2/MW_N2
N_S=m_S/MW_S
x=N_C
y=N_H2
z=N_S
ath=x+0.5*y+z-N_02
w=0.0225+3.76*ath
mprod=x+y+z+w
NCO2=N_C
NH2O=N_H2
NSO2=N_S
NN2=N_N2
MW_CO2=44;
MW_H2O=18;
MW_SO2=64;
MW_N2=28;
YCO2=NCO2/mprod
YH2O=NH2O/mprod
YSO2=NSO2/mprod
YN2=NN2/mprod
Mprod=(N_C*MW_CO2+N_H2*MW_H2O+MW_SO2*NSO2+N_N2*MW_N2)/mprod
mfuel=100;
mair=29*(ath*4.76)
AF=mair/mfuel
Recommended Reading
|
I would like first of all the author for this marvelous piece of work. It's a multi disciplinary book which needs the student to know chemical kinetics and dynamics , mass transfer and fluid mechanics. It's a very easy to read full of solved examples. For a person new in the field he will find it as an easy book to extract what he is looking for. Chapters two gives the impression that the book is not in an organized manner, but once passed the rest seem very well written and outlaid. The summary at the end of each chapter provide a good source of ideas refreshment after going through the chapters. The mass transfer part which is related to the mass diffusion and its relation to multi component diffusion has been covered well. For the student to understand the book he needs to have been exposed to computational fluid dynamics beforehand because that will help him understand the modeling aspects discussed in the book not forgetting the introduction to turbulent flows. The sections that discus the modeling aspects are good as an introduction to the student but not enough for understanding in depth. Chemical kinetics is discussed but you don't feel that it's straight forward to understand especially if your not from a chemical engineering field.
|
The coupling issue of chemical and thermal analysis of reacting systems is discussed in chapter 6 Turbulent premixed combustion and methods of characterization using dimensionless numbers and the use of the Borghi chart was discussed. Laminar premixed flames is discussed thoroughly with the study of the effects of different parameters on flames such as pressure , temperature, ....... etc. The last chapter of the book discuss detonation which is a good introduction for a student who wants to further his knowledge in the field. The appendices provide the student with a rich data base of information which can help him in his course work, lab experiments and research. Radiation and acoustics in combustion weren't allocated any chapters. Hope that was helpful
Unless otherwise noted, all content on this site is @Copyright by Ahmed Al Makky 2012-2018 - http://cfd2012.com